Abstract
Introduction: Chronic red blood cell exchange transfusion (CET) therapy is currently used to prevent neurologic and other complications of sickle cell disease (SCD) in children and adults. Despite past pediatric studies showing that CET decreases stroke risk and hospital admissions, few studies have evaluated whether CET decreases hospital utilization or pain in adults with SCD. Data support health care utilization is a poor marker of pain frequency so other ways of assessing the impact of therapy on pain outcomes is necessary and important. We noted that despite receiving CET, our adult patients experienced daily pain requiring home opioid use and intermittent hospitalization for pain crisis. Thus, we conducted a study to examine the effects of CET on the pain experience. Our primary hypothesis was that adults on CET will have similar levels of pain as defined by Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) pain domains as compared to adults not on CET. Our secondary hypothesis was that adults on CET will have similar levels of hospital utilization, opioid use, QOL measures of domains noted to be affected by SCD or inflammatory markers shown to be correlated with pain and SCD pain crisis.
Methods: We conducted a pilot cross-sectional study of adults with SCD treated with or without CET. Subjects were enrolled at scheduled clinic visits and subjects not at their heathy baseline or who receive regular simple transfusion were excluded. Our primary outcome was pain as defined by ASCQ-Me pain domains which include pain crisis frequency, pain crisis severity, and 7-day pain interference which allow the tool to measure both crisis pain and chronic pain. Our secondary outcomes were hospital utilization, opioid use, QoL measures included in ASCQ-ME: sleep, stiffness, social impact and emotional impact, Patient Reported Outcome Measurement Information System domains for nausea and anxiety and inflammation measured by WBC and platelet counts, C reactive protein (CRP) and cytokine levels. Hospital utilization (total annual admissions and emergency room visits) was assessed by medical record review over a one-year period (1/1/2016 to 12/31/2016). Weekly opioid use was estimated by review of dispensed prescriptions using the Connecticut Prescription Monitoring Program converted into average weekly oral morphine equivalents. Tumor necrosis factor alpha (TNFα), interleukin 1α and 1β (IL-1α, IL-1β) and interleukin 4 (IL-4) were measured using a luminex multiplex assay. Blood was obtained prior to starting CET. The R statistical software program was used to perform t-tests and Wilcoxon rank-sum tests where normality was not met to compare subjects on CET to those not on CET. Significance was set at p<0.05.
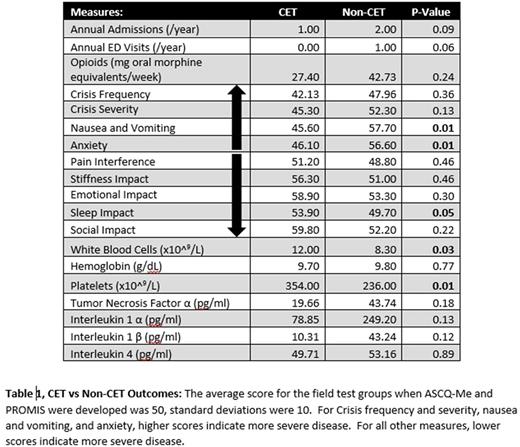
Results: We enrolled 27 subjects (n=16 in no CET group and n=11 in CET group); indications for CET included primary (n=1) or secondary (n=10) stroke prophylaxis. There was no difference between groups in mean age (33.3 SD 10.1 vs 29.4 SD 12.4 years, p=0.4) or gender (% female: 45% vs. 63%, p=0.3). No patients on CET and 68.7% of those not on CET were prescribed hydroxyurea. Median time patients were receiving chronic transfusions was 5 (IQR 2-14.5) years, and median pre-transfusion %S was 40.3 (IQR 32.2-44.9) %. Subjects on CET had similar median admissions and ED visits compared to those not on CET (1 (IQR 0-1.5) vs 2 (IQR 0-5) admissions p=0.09, 0 (IQR 0-0.5) vs 0.5 (IQR 0-2) ED visits p=0.07). Subjects in both groups were dispensed similar median weekly doses of opioids (CET: 27.4 (IQR 3.0-200.5) vs No CET: 42.7 (IQR 13.0-171.6) mg oral morphine equivalents p=0.7). Subjects on CET had similar ASCQ-ME scores to subjects not on CET for pain episode frequency, severity, and 7-day pain impact (Table 1). Those on CET had superior scores for sleep, nausea, and anxiety (Table 1). Subjects on CET had higher baseline platelet and WBC counts and there was no difference in CRP or cytokines measured between groups (Table 1).
Conclusions: Collectively, our pilot data suggest despite patients receiving CET pain could still develop. Thus, additional therapies in combination with CET may be needed to optimize pain and quality of life in adults living with SCD. We may have failed to show a difference in hospital utilization due to our subjects being placed on CET for neurologic events, not frequent crisis. A larger prospective cohort study should be done to confirm our preliminary findings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.